Coagulation Studies
/All bleeding stops…eventually.
True, but let's take a look at why some bleeds take longer to stop than others and more importantly how we gauge which will continue to bleed so we don't get to the PEA end of eventually...
Today the discussion is about coagulation stuides. What are they good for? What aren't they good for? Before we go coag crazy, we are going to do a deep(ish) dive on how coagulation works in the first place, and what it is exactly that our coag tests are measuring.
Conceptually, hemostasis has classically been divided into primary (platelet plug formation) and secondary (factor dependent fibrin clot) mechanisms, with the implication that this is a linear process.
Current thinking approaches this as a more dynamic and interdependent process, with processes occurring synchronously while impacting each other. Since we are emphasizing coagulation, we will omit the discussion of platelet activation (targets for ASA, clopidogrel, ticagrelor) and suffice to say that platelets help make clots (assuming you have enough, and the ones you have are functioning properly).
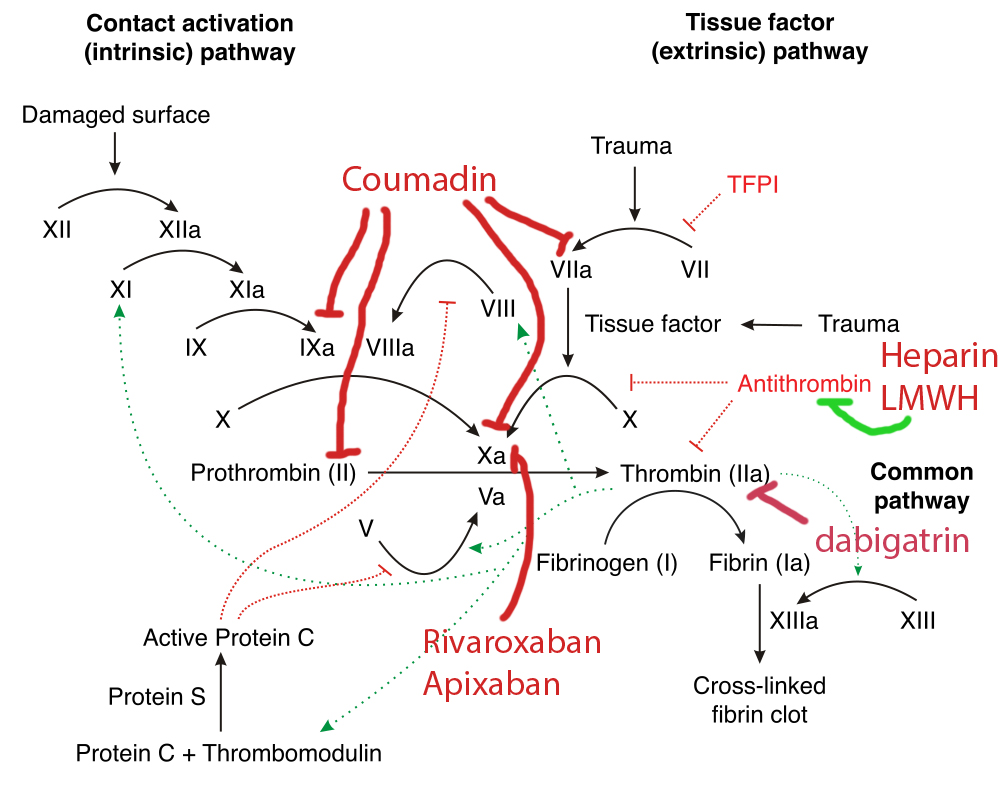
With regards to secondary hemostasis, that is, the infamous coagulation cascade, the following diagram may look familiar:
If that isn’t sufficiently complex, all of these reactions are occurring in a three-dimensional space and require physical substrates (a phospholipid) and cofactors to proceed.
Luckily, the detail in these diagrams can, for our purposes, be distilled down significantly. The so-called “extrinsic” pathway, which involves the complex of tissue factor r(release from damaged endothelium) and factor VII (one of the vit-K dependent factors, along with 2, 9, 10, C and S) is the primary active pathway in vivo. This is measured, as we will discuss below, by the PT. The “intrinsic” pathway requires most of the other factors, and is measured by the PTT. The “common” pathway is the shared downstream portion that starts with factor X->V->II->I. The enzymatic nature of the clotting cascade allows for rapid amplification of this reaction, with downstream factors acting to boost the upstream reaction- creating an exponentially accelerating process. This process does require both a phospholipid physical medium and calcium as a co-factor, which we will use to our advantage to control our laboratory studies.
Now that we have a brief refresher on what the body is doing, let’s delve into the mechanism of our coagulation assays.
Coagulation studies need to be collected in a tube with sodium citrate (light blue top). The citrate in the tube binds calcium, which inactivates the clotting potential. Tubes need to be adequately filled, since an excessive amount of citrate/blood can further inhibit coagulation and skew results- so this isn’t a test we can accurately run with that tiny drop we get before a line blew.
The standard coag panel provides three variables: PT, aPTT and INR.
To analyze Prothrombin Time (PT), the light blue tube is spun down on in a centrifuge and the serum is removed. Calcium is then reintroduced to this tube, along with phospholipid (also needed for the reaction) and tissue factor (which complexes with factor 7 and initiates the “extrinsic” pathway). Once these are added, the cascade begins and leads ultimately to the formation of insoluble fibrin strands. The coagulation of the serum can be detected optically or mechanically, depending on the lab equipment. The time required for this change to occur is the PT. Of note, the tissue factor or “extrinsic” pathway is the primary mechanism for coagulation in vivo (observe that the PT values are much lower than the aPTT). The original conceptual distinction between “intrinsic” and “extrinsic” is an artifact from these lab tests.
The PT is then adjusted based on various laboratory agents used to calculate the International Normalized Ratio (INR), a standardized value that allows comparison between lab data generated from other machines, laboratories or hospitals. A PT from our hospital and the PT from an OSH may differ significantly for the same sample of blood, but the INR should be the same. INR is most commonly used to monitor warfarin dosing, and is preferred over the aPTT because factor 7 has the shortest half-life of the pro-thrombotic factors, and thus PT is more responsive to changes in vit-K dependent factor synthesis.
The activated Partial Thromboplastin Time (aPTT), is obtained using in a nearly identical process to the PT, except that only phospholipid, calcium and ground glass (an inert physical activator) are added to the reaction, so the factor 7-tissue factor pathway is not activated. The aPTT is altered by changes in all of the clotting factors (except 7), and is frequently used to monitor Heparin dosing (Hep activates antithrombin 3, which will inhibit all clotting factors except 5 and 8).
Now that we’ve reviewed the clotting cascade and how our lab values are derived, let's contextualize this with the therapies we use in practice by the diagram on the right. Stay tuned for more cases this week in the Grand Rounds Recap.
Post by Aaron Murphy-Crews, MD
Peer Review and Editing by Ryan LaFollette, MD