Annals of B Pod - Hypertension in Pregnancy
/HISTORY OF PRESENT ILLNESS
A pregnant female in her early 30s with no recorded prenatal care was found unresponsive by Emergency Medical Services (EMS) at home thirty minutes after an unwitnessed event. The patient received two doses of intranasal naloxone on scene with minimal response. Upon arrival to the Emergency Department (ED), a Maternal-Fetal Medicine (MFM) physician performs a bedside ultrasound, which dates the pregnancy at approximately 28 weeks gestation and confirms intrauterine fetal demise (IUFD). The patient is intubated for respiratory failure, given two units of packed red blood cells for profound anemia, and started on norepinephrine for blood pressure support.
Past medical history: IV drug use, hepatitis C
Past obstetric history: Placental abruption at 28 weeks in last pregnancy, Rh isoimmunization
Past surgical history: C section, back surgery
Medications: None
Allergies: No known allergies
PHYSICAL EXAM
Pre-resuscitation Vitals: T 36.5 HR 118 BP 50/palp RR 28 SpO2 55% GCS 3
Post-resuscitation Vitals: T 35.8 HR 109 BP 145/83 RR 22 SpO2 100% GCS 3
The patient is a woman who is intubated and sedated. Her pupils are sluggishly reactive. She is tachycardic but heart sounds suggest a regular rhythm with no appreciable murmurs. On lung exam, she has bilateral breath sounds with no wheezes, rhonchi, or rales. Abdominal exam reveals a gravid uterus with the fundus measuring 6 cm above the umbilicus, and her prior cesarean incision is noted. There is no vaginal bleeding noted on external evaluation; bimanual examination reveals cervical dilation at 1 cm. Her extremities are cold, clammy, and pale. The patient is unresponsive to painful stimuli.
Diagnostics
WBC: 19.0 Hgb: 6.2 Hct: 20 Plt: 184
Na: 140 K: 4.1 Cl: 105 HCO3: 8 BUN: 8 Cr: 1.0 Glucose: 329
ABG: pH 6.74 pCO2 61 pO2 303 BE -29
Lactate: 19.2
Alk Phos 111 AST 63 ALT 17 T Bili 0.2
INR 2.4 PT 26.8 Fibrinogen 93
Uric Acid 8.3
Peripheral Blood Smear: normocytic anemia, thrombocytopenia consistent with hemolytic/microangiopathic anemia
Rapid Thromboelastography (TEG): ACT 191s R Time 90s K Time 250s Angle 55° Max amplitude 48mm Lysis 30.0%
CK 1,284 Trop 0.094
Kleihauer-Betke: 0% fetal maternal hemorrhage
Urine Protein : Creatinine Ratio 34.0
Urine Drug Screen: Negative
CT/CTA head and neck: No acute intracranial abnormalities
EEG: no focal, lateralizing or epileptiform features appreciated
CT Abdomen and Pelvis: Intrauterine pregnancy, large placental abruption measuring 17.7 x 13.4 x 16.5cm
Obstetric Ultrasound: confirmed IUFD, cephalic presentation, no hydropic changes, complete placenta abruption noted
Hospital course
After initial resuscitation with blood product transfusion, vasopressors, and mechanical ventilation, the patient was noted to have systolic pressures of greater than 140mmHg, which was concerning for preeclampsia in the setting of pregnancy. She was transferred to the Surgical Intensive Care Unit (SICU) for continued management where induction of labor for IUFD was initiated. Shortly after transfer to the SICU, seizure activity was noted. Given the potential for eclamptic etiology, the patient was treated with intravenous lorazepam 2 mg, followed by a bolus of intravenous magnesium sulfate 6 g followed by a continuous infusion of 2 g/hour. Ultimately, the patient completed a spontaneous vaginal delivery of a phenotypically normal-appearing demised male infant. The delivery was complicated by postpartum hemorrhage of approximately 1400 mL, consistent with concealed placental abruption. The patient was extubated on postpartum day one and treated with magnesium sulfate for twenty-four hours post-delivery. Her coagulopathy was corrected by TEG-directed blood product replacement. After transfer out of the SICU, the patient proceeded to have a relatively uncomplicated postpartum course with discharge from the hospital.
Preeclampsia and Eclampsia
Definitions and Epidemiology
Preeclampsia is defined as hypertension plus proteinuria, or in the absence of proteinuria, signs and symptoms consistent with significant end-organ damage. Preeclampsia is believed to stem from endothelial dysfunction, possibly secondary to impaired placentation early in gestation, resulting in impaired balance of proinflammatory and antiangiogenic factors that worsens with increasing gestational age.[1] Although not entirely understood, this dysregulation is thought to progress and result in cerebral vasospasm leading to new onset generalized tonic-clonic seizures during pregnancy, formally known as eclampsia.[1,2] As such, preeclampsia is traditionally thought to be one part of a spectrum of disease, and if left undetected or untreated, it can progress to eclampsia. However, 25% to 40% of eclampsia cases are documented to occur in the absence of premonitory signs of preeclampsia. [3,4] Even in eclampsia cases that are preceded by elevated blood pressures, 33% of these cases have only mild elevation in blood pressures, less than the classic threshold of 160mmHg/110mmHg. Thus, this remains a difficult disease process to diagnose.
Hypertensive disorders of pregnancy are amongst the most common obstetric emergencies, and represent some of the most significant causes of maternal and neonatal morbidity and mortality. [2] Among cases of eclampsia, studies show that approximately 23% of patients require mechanical ventilation and 35% have at least one other major complication such as the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP); pulmonary edema; acute respiratory distress syndrome (ARDS); disseminated intravascular coagulation (DIC); renal failure; stroke; or cardiac arrest. [4] Risks to the neonate include premature birth, placental abruption, and growth restriction. [2] Furthermore, studies have demonstrated that women with hypertensive disorders during pregnancy have an increased risk for IUFD, although the pathophysiology is not fully understood. [2,5] Up to 33% of eclamptic seizures occur outside of the hospital, making early recognition of predisposing risk factors essential. [4] Emergency providers should have a high suspicion of eclampsia in any critically ill, pregnant patient who presents to the ED or requires admission to the ICU. [4,6]
Clinical Presentation
Pregnant patients presenting with headache, visual disturbances, abdominal pain, or shortness of breath should raise the suspicion for preeclampsia. However, patients can oftentimes present with asymptomatic hypertension; thus, blood pressure monitoring is paramount during pregnancy. In patients presenting with altered mental status or seizure activity, eclampsia should be suspected and managed quickly.
Diagnostic Considerations
The differential diagnosis for eclampsia can be vast, and other etiologies of altered mental status or seizure activity should be considered, including trauma, metabolic derangements, toxic exposure or ingestion, neurologic insults, and infectious etiologies.
In regards to diagnosing hypertension during pregnancy, the classic definition is systolic blood pressure greater than 140mmHg or diastolic blood pressure greater than 90mmHg on at least two occasions, or at least four hours apart, after a female has reached twenty weeks of gestation. [2] If this is new onset hypertension in the setting of pregnancy, this is termed gestational hypertension. If hypertension occurs prior to this gestational age, the patient likely has had pre-existing hypertension, and this also needs to be similarly managed during pregnancy. It is only if a patient has proteinuria or other signs or symptoms of end-organ damage that she is diagnosed with preeclampsia.
In addition to a detailed neurologic exam to assess for visual or cerebral symptoms, laboratory analysis is the cornerstone for diagnosing preeclampsia. A urinalysis is needed to assess for proteinuria (≥0.3 g in a 24-hour urine specimen or protein/creatinine ratio ≥0.3), a complete blood count to assess for anemia indicative of hemolysis or thrombocytopenia, a renal panel to assess for renal insufficiency, a hepatic panel to assess for elevated liver enzymes, a uric acid and lactate dehydrogenase to assess for hemolysis, and a chest X-ray or ultrasound to assess for pulmonary edema in the appropriate clinical settings. [2] An electroencephalogram can also be used if presentation is ambiguous for neurologic versus non-neurologic etiology. The combination of blood pressure measurement and the diagnostic evaluation can help determine the severity of the patient’s condition and the necessary treatment steps to follow.
Treatment
Delivery is the only curative therapy for severe preeclampsia and eclampsia, but neonatal viability must be weighed into this decision in concert with an obstetric specialist. [7] Additionally, the mother must be stabilized first and foremost prior to consideration for delivery and transport to a facility with appropriate resources. Thus, as with all other emergency management, assessing maternal airway, breathing, and circulation is paramount, along with limiting maternal trauma sustained from the seizure episode. Placing the mother in the left lateral decubitus position will assist in relieve pressure off of the inferior vena cava, and thus improve venous return and maternal circulation.
Next, prioritization of treatment of recurrent seizures needs to occur. Magnesium sulfate is the treatment for preventing recurrent eclamptic seizures and has been used since the early 1900s. While not traditionally considered an anticonvulsant, magnesium sulfate is effective due to the calcium channel antagonism, leading to smooth muscle relaxation and reversal of cerebral arterial vasospasm. [8] It also corrects the underlying endothelial injury that is characteristic of this disease state by releasing endothelial prostacyclin and simultaneously inhibiting platelet aggregation. [9] Magnesium has been shown to be more effective than phenytoin and diazepam for the treatment of seizures in patients with eclampsia, and often can be used successfully as a single agent therapy in this patient population. [10] A loading dose of 4-6 grams of IV magnesium should be administered over thirty minutes, followed by a continuous infusion of 1-2 grams of IV magnesium per hour. [10] Only if the patient continues to have refractory seizures should other antiepileptic medications be considered. Patients with renal dysfunction may have an increased risk for supratherapeutic magnesium levels, and therefore increased monitoring may be warranted targeting a goal of goal of greater than 2-4 mg/dL.[11] Patients should also be monitored for signs or symptoms of toxicity, such as increased hypotension and respiratory depression. Calcium gluconate may be used as an antidote for magnesium toxicity.
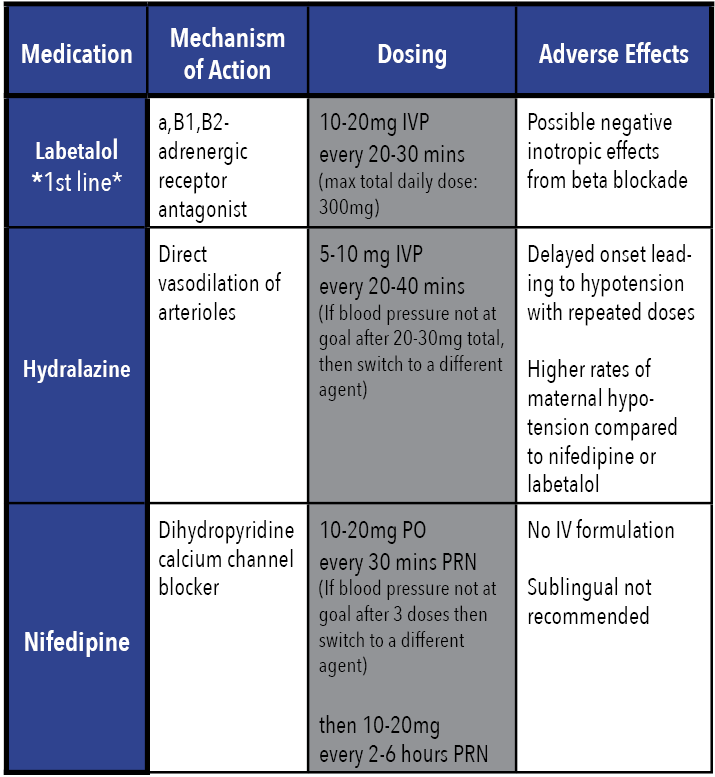
In conjunction with seizure management, severe hypertension must also be emergently controlled to prevent risk of stroke and intracranial hemorrhage. The first line agents for blood pressure control are IV labetalol, IV hydralazine, or PO immediate release nifedipine.[10,12] The goal is not to normalize blood pressure, but to prevent prolonged exposure to severe systolic hypertension; thus, an initial goal range of 140–150mmHg systolic pressure and 90–100 mmHg diastolic pressure is reasonable. [7]
Prognosis
Maternal mortality rates of up to 14% have been documented in eclampsia, with up to 15% to 20% of deaths attributable to stroke secondary to severe, uncontrolled hypertension. [13] If the mother survives, there are future consequences to both maternal health and complications with future pregnancies. Women with a history of eclampsia have demonstrated to be at an increased risk for cardiovascular and cerebral disease lateral in life, with chronic hypertension being a commonly developed co-morbidity. [14] Furthermore, recurrent eclampsia occurs in about 2% of subsequent pregnancies.[14] Even if maternal hypertension is closely monitored and managed in future pregnancies, there continues to be an elevated risk for placental abruption, preterm delivery, fetal growth restriction, and perinatal mortality. [14] Thus, all future pregnancies should be monitored with a maternal-fetal medicine specialist as they are considered to be high-risk.
Summary
Preeclampsia and eclampsia hold high maternal and neonatal morbidity and mortality rates, and require prompt recognition. A high degree of suspicion must be held in any critically ill pregnant patient more than twenty weeks of gestation, and quick initiation of appropriate management, including maternal stabilization, management of recurrent seizures, and adequate control of hypertension, is vital in reducing risk for complications.
AUTHORED BY jessica gottula, MD
Editing BY the Annals of B Pod Editors
References
Sanfilippo JS, Rock JA. Surgery for benign disease of the ovary. In: TeLinde's Operative Gynecology, 11th ed., Jones HW, Rock JA (Eds), Wolters Kluwer, 2015.
Pelvic Mass. In: Hoffman BL, Schorge JO, Bradshaw KD, Halvorson LM, Schaffer JI, Corton MM. eds. Williams Gynecology, 3e New York, NY: McGraw- Hill. http://accessmedicine.mhmedical.com/content.aspx?bookid=1758§ionid=118168387. Accessed July 06, 2019.
Varras M, Tsikini A, Polyzos D, et al. Uterine adnexal torsion: pathologic and gray-scale ultrasonographic findings. Clin Exp Obstet Gynecol 2004; 31:34.
Oltmann SC, Fischer A, Barber R, et al. Cannot exclude torsion--a 15-year review. J Pediatr Surg 2009; 44:1212.
Pansky M, Smorgick N, Herman A, et al. Torsion of normal adnexa in postmenarchal women and risk of recurrence. Obstet Gynecol 2007; 109:355.
Houry D, Abbott JT. Ovarian torsion: a fifteen-year review. Ann Emerg Med 2001; 38:156.
Yen CF, Lin SL, Murk W, et al. Risk analysis of torsion and malignancy for adnexal masses during pregnancy. Fertil Steril 2009; 91:1895.
Huchon C, Fauconnier A. Adnexal torsion: a literature review. Eur J Obstet Gynecol Reprod Biol 2010; 150:8.
White M, Stella J. Ovarian torsion: 10-year perspective. Emerg Med Australas 2005; 17:231.
.Huchon C, Panel P, Kayem G, et al. Does this woman have adnexal torsion? Hum Reprod 2012; 27:2359.
Rousseau V, Massicot R, Darwish AA, et al. Emergency management and conservative surgery of ovarian torsion in children: a report of 40 cases. J Pediatr Adolesc Gynecol 2008; 21:201.
Rossi BV, Ference EH, Zurakowski D, et al. The clinical presentation and surgical management of adnexal torsion in the pediatric and adolescent population. J Pediatr Adolesc Gynecol 2012; 25:109.
Pour, TR and Tibbles CD. (2018). Selected Gynecologic Disorders In R.M. Walls (Ed.), Rosen’s Emergency Medicine: Concepts and Clinical Practice, 9th edition (pp. 1232-1239). Philadelphia, PA: Elsevier.