Annals of B Pod - B Pod Case: Taking Renal Failure to Heart
/The patient is a male in his late 30s with a past medical history significant for trisomy 21, stage III chronic kidney disease of unspecified etiology, and hypertension who presents to the Emergency Department with emesis and dark stools. The patient is unable to contribute significantly to his history, but his family relates that two days prior to presentation, the patient experienced two episodes of “coffee ground” emesis according to the patient’s home health nurse. Over the next day, the patient subsequently experienced several episodes of melenic stools. His family also notes that he has seemed feverish, more lethargic, and less active than his baseline.
Vitals: T 37.2 HR 125 BP 183/108 RR 14 SpO2 97% on RA
Physical Exam: Cardiovascular examination is notable for tachycardia with a regular heart rhythm. A grade III/VI systolic murmur is best appreciated over the left parasternal border without audible gallops, murmurs or rubs.
His abdominal exam is normal without organomegaly, focal tenderness, or guarding. Digital rectal examination was performed, which revolved guaiac-positive, black stool in the rectal vault. He has evidence of bilateral lower extremity pitting edema. The patient is lethargic but oriented to person, place, time, and purpose with no gross neurological deficits. His speech is clear without aphasia or dysarthria, and his skin is warm and dry without diaphoresis.
Diagnostics
WBC: 10.7 Hgb: 4.9
Na+: 131 K+: 5.7 BUN: 153 Creatinine: 24.63
Fecal occult: Guaiac +
Chest x-ray: Cardiomegaly with patchy consolidation in the right lower lobe concerning for pneumonia.
Cardiac ultrasound: Evidence of moderate to large pericardial effusion
Hospital Course
This patient presented with coffee-ground emesis, melenic stools, and a low hemoglobin concerning for a significant upper gastrointestinal hemorrhage. His diagnostic evaluation also showed acute on chronic renal failure with significant uremia, as well as a new pericardial effusion. Given the severity of his GI bleed, the patient was transfused with four units of packed red blood cells in the Emergency Department prior to transfer to the Medical Intensive Care Unit for further management.
Upon admission, the patient’s family was informed that the patient would likely need hemodialysis given the severity of his renal failure. Though noted to have a pericardial effusion, the patient remained hemodynamically stable with no evidence of tamponade. As such, pericardiocentesis was not deemed a priority given the patient’s more critical comorbidities. The admitting team also recommended that the patient undergo esophagogastroduodenoscopy (EGD) to evaluate for the source of his hemorrhage.
In discussing the potential complications of EGD, the likely need for intubation, and the patient’s likely need for lifelong hemodialysis, his family ultimately requested that patient be discharged home on hospice. The patient passed away at home roughly two weeks after discharge from the hospital.
Pericardial Effusion
A pericardial effusion is an abnormal collection of fluid within the pericardial sac. The pericardial sac is a potential space formed between the visceral and parietal layers of the fibrous covering of the heart. This potential space normally contains a small volume (~50 mL) of serous fluid similar in composition to plasma ultrafiltrate that serves as a lubricant, allowing the two layers to slide over one another during the movements of the heart.[1] The volume of this pericardial fluid is regulated by various lymphatic systems that drain the pericardium.
The onset of a pericardial effusion can be categorized as acute (≤1 week), subacute (>1 week and ≤3 months), and chronic (>3 months).[2] Similarly, the size of an effusion may be categorized semiquantitatively using ultrasonography by measuring the diameter between the visceral and parietal pericardial tissue in diastole (smallest size during cardiac cycle while in M Mode).
Various perturbations in normal physiology or exogenous insults (e.g., infections) may lead to inflammation of the pericardial tissue (pericarditis) or a fluid collection in this potential space that exceeds the lymphatic drainage. Rapid accumulation of fluid may lead to “tamponade” physiology, wherein the collection of material within the inflexible pericardium may lead to a build up of pressure that exceeds the resilience of the ventricular walls. This can limit diastolic filling and thus decrease stroke volume and cardiac output, with consequential hemodynamic instability. Fluid that accumulates more insidiously, however, may lead to a gradual dilation of the pericardial sac. Volumes exceeding 2L of fluid may accumulate via this gradual dilatation prior to the onset of tamponade physiology. [2.3]
Detailing the chronicity of pericardial effusions is frequently complicated by their often subtle presentations. Perhaps more meaningful, then, is to determine evidence of hemodynamic instability or compromise secondary to an effusion. Common physical exam findings in patients with significant effusion or tamponade physiology include dyspnea, tachycardia, and hypotension. Patients may present with jugular venous distention or pulsus paradoxus (a drop in systolic blood pressure >10 mmHg during inspiration). The presence of elevated jugular pressure, hypotension, and muffled heart sounds is known as Beck’s triad and is pathognomonic for cardiac tamponade.
Electrocardiography is often a useful adjunct in the diagnosis of pericardial effusion. Wide-spread, upward-concave ST segment elevations may be indicative of significant peridcardial inflammation.[4] Diffuse low-voltage lead tracings may be suggestive of a large pericardial effusion, whereas electrical alternans is often cited as pathognomonic for a large pericardial effusion or tamponade. Literature indicates that the sensitivity of these findings, however, is quite poor.[5]
Echocardiography may also be used in the diagnosis of tamponade physiology. Findings suggestive of tamponade physiology include collapse of any of the cardiac chambers (most often the right atrium and ventricle, due to their comparatively thinner, less muscular walls) and inferior vena cava plethora (>20mm).[2,6] The most specific marker for tamponade is diastolic collaspe of the right atrium.[2]
As mentioned previously, pericardial effusions may stem from a number of etiologies including numerous infectious agents (tubercular, bacterial, viral, fungal, parasitic), autoimmune processes, postinfarction inflammation, malignant effusions, traumatic (pneumo- or hemopericardium), and metabolic derangements (including uremia).[2] The incidence of these diseases varies dramatically on the country and demographics examined.
Uremic Pericardial Effusion
Uremic pericarditis and pericardial effusion, as seen in this patient, are well-known complications of azotemia, though the exact pathophysiologic mechanism is poorly understood. Uremia refers to an abnormally high level of urea (BUN >60 mg/dL) and other nitrogenous waste products retained in the blood. The kidneys play an essential role in the elimination of nitrogenous waste from the blood and, as a result, uremia invariably stems from renal dysfunction.
There are numerous nephropathies that may lead to progressive renal dysfunction and uremia. Interestingly, the incidence of pericarditis and pericardial effusion correlates with the severity of a patient’s uremia/azotemia and not the etiology of patient’s kidney disease.7 It has been speculated that platelet dysfunction and consequential coagulopathy in uremic patients may in part contribute to pericardial effusions, though the pathogenesis is again poorly understood.[8]
Pericardial effusion is a clinical diagnosis, though confirmation studies with echocardiography have become the standard of care. Patients presenting with symptoms concerning for pericardial effusion may be evaluated with a complete blood count, basic metabolic panel, thyroid function, electrocardiography, and plain films of the chest.[4] Erythrocyte sedimentation rates and C-reactive protein may be elevated in the event of inflammatory processes.
Serum troponin levels may be elevated in patients with significant pericardial inflammation, large effusions, traumatic injuries or tamponade physiology leading to myocardial injury.9 Furthermore, rheumatologic testing may be indicated in young female patients due to the higher incidence of autoimmune disease. Fluid samples collected from the pericardium may be sent for Gram staining, bacterial/fungal cultures, acid-fast staining and/or mycobacteria-specific cultures, tailored to patient’s clinic presentation and regionally-endemic pathogens.
Emergent pericardiocentesis performed in the Emergency Department under ultrasound guidance is indicated in patients with evidence of severe hemodynamic instability (cardiac tamponade).6 Approaches to pericardiocentesis are varied; the most common approaches include parasternal or subxiphoid needle insertion with transthoracic echocardiographic guidance. Ultrasonography should be used in all emergent cases, as blind pericardiocenteses are associated with a high rate of complications (>20%), including pneumo- or hemothorax, laceration of the coronary arteries, puncture or rupture of the ventricular walls, and injury to the thoracic vasculature (e.g., internal mammary and intercostal arteries).6,10 Large but hemodynamically stable effusions may be drained nonemergently under fluoroscopic guidance or by cardiothoracic surgery. In both instances, fluid analysis may be performed to determine the etiology of the effusion.
Small or moderate sized effusions without tamponade physiology may be managed pharmacologically. Good evidence exists for the use of anti-inflammatory agents in both treating and preventing recurrence of effusion.[2] Various drug classes, including NSAIDs (specifically ibuprofen and aspirin), corticosteroids (prednisone), and atypical agents (such as colchicine), have relatively robust evidence for their use. Generally, first line treatment involves the scheduled use of nonsteroidal anti-inflammatory drugs with subsequent assessment to verify remission or resolution of the effusion.[2] In addition, patients should receive appropriate treatment for the underlying pathophysiologic process, e.g., active infection, malignancy, or rheumatologic disease.
Many of the above interventions — specifically NSAIDs and corticosteroids — have demonstrated somewhat mixed results in patients with uremic pericardial disease.[11,12] Current evidence supports the use of dialysis (to remove nitrogenous waste) and colchicine in the management of pericardial effusions in patients with renal disease.[13,14] Unfortunately, recurrence is common in patients with pericardial effusion, particularly in patients with uremia. Recurrent disease may require more aggressive management, including chronic immunomodulation via prolonged steroid or colchicine use, creation of a pericardial window, and pericardiectomy.[14]
In sum, pericardial effusions are multifactorial in etiology and can have varying clinical presentations. Emergency Physicians should have a low threshold to evaluate for recurrent disease in patients with a history of pericardial effusion presenting with concerning symptoms, or in any patient with risk factors and concerning symptoms or pertinent physical exam findings.
Procedure: Pericardiocentesis
As fluid accumulates in the pericardial space, it exerts pressure on the heart, ultimately resulting in equalization of diastolic filling pressures as it continues to progress. This can impair ventricular filling and may eventually result in decreased cardiac output leading to pulseless electrical activity and death. Pericardiocentesis can be a life-saving procedure in patients with such presentations.
In the emergency department setting, it is indicated in hemodynamically unstable patients with evidence of tamponade[15], whether that is on bedside ultrasound or clinically with Beck’s triad (hypotension, distended neck veins, and distant heart sounds) or pulsus paradoxus (inspiratory decrease in systolic blood pressure). Classically, this procedure is relatively contraindicated in traumatic pericardial effusions, myocardial rupture, serious bleeding disorders, and aortic dissection due to concerns for rapid reaccumulation of the effusion. However, in hemodynamically unstable patients, there are no absolute contraindications to performing this procedure as it may be the only available treatment for life-threatening pericardial effusion. Nonetheless, it should be considered a temporizing measure, and definitive treatment for the underlying etiology of the effusion should be pursued.
Supplies
- Sterile gloves
- Betadine (or other similar cleaning solution)
- 18 gauge spinal needle
- 20 cc syringe
- 3-way stop-cock
- Ultrasound (if available)
Procedure
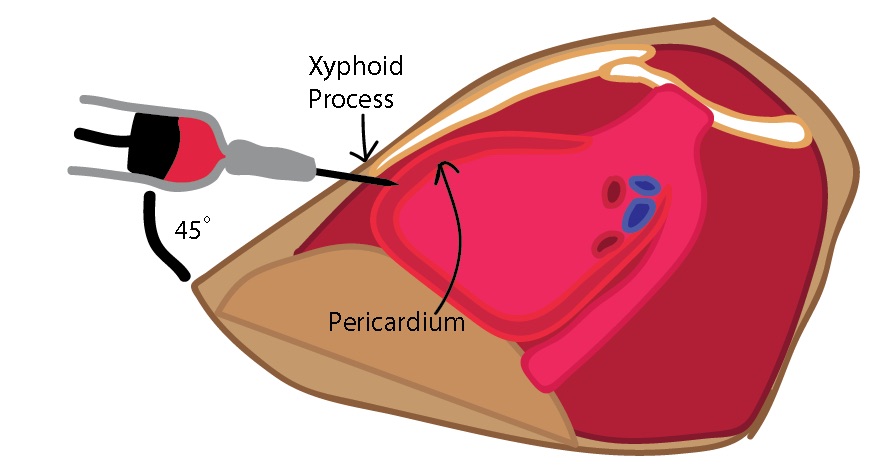
- Cleanse a wide area of skin just above the xiphoid process using Betadine or other cleansing solutions.[16]
- Palpate the xiphoid process to determine landmarks. Visualize the effusion if using ultrasound.
- Insert the spinal needle with the stylet in place just inferior to the xiphoid process. Aim superiorly and angled toward the left shoulder.
- Once the skin has been punctured, remove the stylet and attach the stop-cock and 20 cc syringe to the spinal needle.
- Advance the needle at a 45 degree angle while aspirating toward the largest fluid collection if using ultrasound.
- If available, IV tubing may be attached to the third port on the 3-way stop-cock in order to allow quick access for additional fluid drainage if necessary.
- Withdraw fluid until hemodynamic stability is obtained, and then turn the stop-cock toward the needle to stop the flow of fluid.
- At this point, the syringe may be removed. Keep the needle and stop-cock assembly in place so that additional fluid may be drained if the patient again becomes hemodynamically unstable.
- After the procedure, it is recommended to obtain an ultrasound to evaluate for improvement in cardiac function in addition to a chest x-ray to evaluate for pneumothorax.
Need to KNow
While pericardiocentesis can be a life-saving procedure, it is not without potentially dangerous complications, which occur in up to 10% of all cases of pericardiocentesis.[17] There is a 20% rate of life-threatening complications if this procedure is performed without ultrasound guidance, but that number is reduced to 3% with the use of ultrasound, making its use the standard of care. Cardiac puncture occurs in 1% of cases and may be subtle, resulting in a persistent effusion and potential redevelopment of tamponade. Management frequently necessitates involvement of cardiac surgery for repair.
Misdirection of the needle through the pleural space can result in pneumothorax or pneumopericardium, both of which may be detected on the post-procedure chest x-ray. Chest tube placement may be indicated in the case of pneumothorax, while close observation is often sufficient for pneumopericardium because it often spontaneously resolves.[17]
Pericardial decompression syndrome is a term used to describe the development of pulmonary edema after pericardiocentesis, a phenomenon which is seen in patients with both acute and chronic pericardial tamponade. It is thought to be due to a greater increase in the right ventricular end-diastolic volume when compared to the left ventricular end-diastolic volume after the procedure.[17] One study showed that after pericardiocentesis, right ventricular stoke volume increased an average of 76%, while left ventricular stroke volume increased by only 64%. This is thought to result in a disproportionate increase in pulmonary blood flow resulting in pulmonary edema. The management of this syndrome is supportive.
Finally, iatrogenic injury to surrounding structures such as the liver, diaphragm, and stomach are not uncommon, highlighting the importance of ultrasound guidance. Thus, Emergency Department pericardiocentesis is often reserved for hemodynamically unstable patients for whom surgical treatment is not readily available. Even so, it can provide life-saving time to transport such patients to definitive care, making it an important skill for the emergency physician.
"Uremic Pericardial Effusion" Authored by Matthew Scanlon, MD
"Procedure: Pericardiocentesis" Authored by Jessica Merriam, MD
Posted by Grace Lagasse, MD
References
- Ben-Horin, S., Shinfeld, A., Kachel, E., Chetrit, A., & Livneh, A. (2005). The composition of normal pericardial fluid and its implications for diagnosing pericardial effusions. The American Journal of Medicine, 118(6), 636-640.
- Imazio, M., & Adler, Y. (2012). Management of pericardial effusion. European Heart Journal, 34(16), 1186-1197.
- Kabukcu, M. et al. Pericardial Tamponade and Large Pericardial Effusions. Texas Heart Institute Journal, 31(4), 398-403.
- Khandaker, M. H., Espinosa, R. E., Nishimura, R. A., Sinak, L. J., Hayes, S. N., Melduni, R. M., & Oh, J. K. (2010). Pericardial Disease: Diagnosis and Management. Mayo Clinic Proceedings, 85(6), 572-593.
- Eisenberg, M. J., Romeral, L. M., Heidenreich, P. A., Schiller, N. B., & Evans, G. T. (1996). The Diagnosis of Pericardial Effusion and Cardiac Tamponade by 12-Lead ECG. Chest, 110(2).
- Jung, H. (2012). Pericardial Effusion and Pericardiocentesis: Role of Echocardiography. Korean Circulation Journal Korean Circ J, 42(11), 725.
- Alpert, M. A., & Ravenscraft, M. D. (2003). Pericardial Involvement in End-Stage Renal Disease. The American Journal of the Medical Sciences, 325(4), 228-236.
- Weigert, A. L., & Schafer, A. I. (1998). Uremic Bleeding: Pathogenesis and Therapy. The American Journal of the Medical Sciences, 316(2), 94-104.
- Brandt, R. R., Filzmaier, K., & Hanrath, P. (2001). Circulating cardiac troponin I in acute pericarditis. The American Journal of Cardiology, 87(11), 1326-1328.
- Fowler, N. O. (1993). Cardiac tamponade. A clinical or an echocardiographic diagnosis? Circulation, 87(5), 1738-1741.
- Spector, D., Alfred, H., Siedlecki, M., & Briefel, G. (1983). A controlled study of the effect of indomethacin in uremic pericarditis. Kidney International, 24(5), 663-669.
- Shabetai, R. (2008). Corticosteroids for Recurrent Pericarditis: On the Road to Evidence-Based Medicine. Circulation, 118(6), 612-613.
- Flather, M. (2006). First line treatment with colchicine reduced recurrent pericarditis. Evidence-Based Medicine, 11(2), 44-44.
- Soler-Soler, J. (2004). Relapsing pericarditis. Heart, 90(11), 1364-1368.
- Adler Y, Charron P, Imazio M, Badano L, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. European Society of Cardiology, 2015. 2921-2964.
- Fitch M, Nicks B, Pariyadath M, McGinnis H, et al. Emergency pericardiocentesis. The New England Journal of Medicine, 2012. 366: e17.
- Kumar R, Sinha A, Lin M, Uchino R, et al. Complications of pericardiocentesis: A clinical synopsis. International Journal of Critical Illness & Injury Science, 2015. 5(3):206-212.
- Lee T, Ouellet J, Cook M, Schreiber M, et al. Pericardiocentesis in trauma: A systematic review, 2013. 75(4): 543-549.